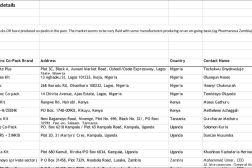
Welcome to the new hub for advocacy and information on co-packaged ORS and Zinc. As of December 9th, 2024, the ORS/Zinc Co-pack Alliance (ORSZCA) has officially joined the Child Health Task Force.
"We want co-packaged ORS and Zinc to become the ‘go-to’ treatment for childhood diarrhea in Low and Middle Income Countries (LMICs)."
– Simon Berry, co-founder of Cola Life and the ORS/Zinc Co-pack Alliance (ORSZCA, now part of the Child Health Task Force)

What is ORS/Zinc Co-Packing?
Oral rehydration salts (ORS)/Zinc Co-Packing is the combination of two effective treatments for diarrhea into a single package. Wide adoption of ORS and Zinc has the potential to prevent more than 90% of the estimated 500,000 annual child deaths from diarrhea, as well as reduce stunting, and improve the quality of millions of children’s lives every year.
70% of child diarrhea deaths are currently occurring in just ten countries, including Nigeria (133,000), India (55,000), Pakistan (32,000), Chad (25,000), Ethiopia (25,000), Niger (23,000), Democratic Republic of Congo (19,000), Cameroon (13,000), Madagascar (11,000), and Somalia (9,500), according to the Global Burden of Disease 2019.
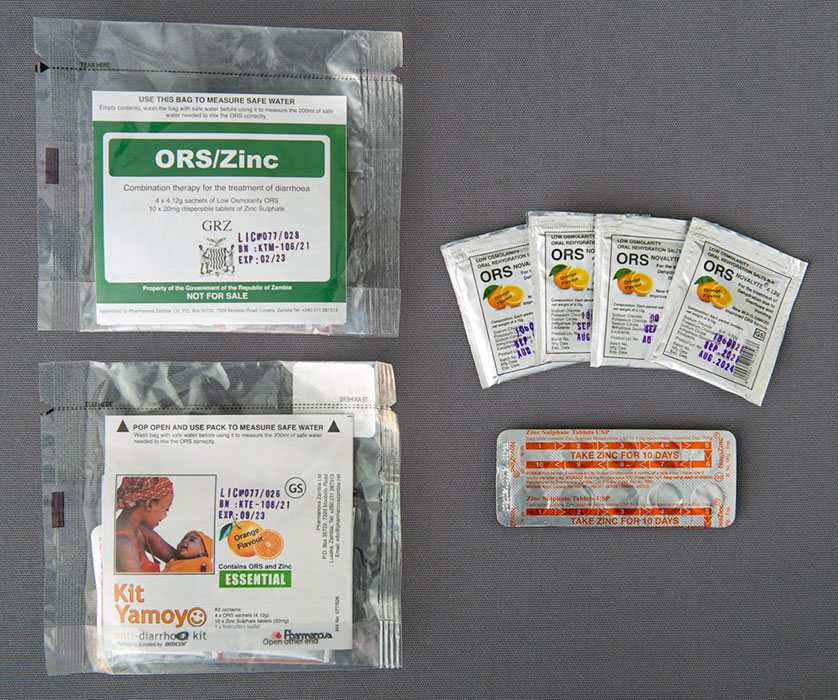

The global recommendation that children should receive ORS and Zinc was published in 2004. Seventeen years later the coverage of ORS and Zinc is just 15% across the countries where data is available. We hope to ensure that no similar delay occurs with adoption of the WHO recommendation that ORS and Zinc should be co-packaged.
Each year of delay in the uptake of ORS and Zinc means more children will die, especially in the countries above, where urgent action is needed. The announcement of the Global Financing Facility (GFF) goal to increase access to diarrhea treatment for 458 million additional children by 2025, as outlined in the Reclaim the Gains campaign, is a major opportunity to reach these children as all of these countries are GFF-eligible.
Current Calls to Action
We have two current calls to action for ORS and Zinc co-packing. Please find more information about them below.
- 1. Advocating for and supporting WHO/UNICEF to issue a joint policy brief on co-packaged ORS and Zinc.
The lack of engagement by UNICEF and WHO following the addition of co-packaged ORS and Zinc to the WHO Essential Medicines Lists in 2019. CHTF believes that a joint statement (or policy briefing) on co-packaging would greatly increase awareness of co-packaging and its benefits, the most significant of which is that co-packaging increases coverage of the recommended treatment for diarrhea (ORS and Zinc) and saves lives.
The objective of such a policy briefing, which would go to all WHO and UNICEF Country Offices, would be to raise awareness of the new co-packaging recommendation in the expectation that this would help with the institutionalization of co-packaging at the country level.
WHO and UNICEF representatives met initially to discuss this call for action on 16-Feb-2023 and subsequently ORSZCA met with the WHO representative on 22-Feb-2023. Following this meeting ORSZCA produced a Position Paper on: Defining an ORS/Zinc ‘co-pack’. On 28 April 2024 WHO/UNICEF posed a set a questions in relation to a joint policy brief which ORSZCA answered in May-2024. These have been commented on by WHO and UNICEF and responses were issued to these comments.
Do you want to contribute? We have a template letter available for download. Send a letter to the director of the UNICEF Supply Division showing support for a statement on co-pakcing ORS and Zinc.
- 2. Participating in the upcoming review of the DHS Questionnaires with the objective of creating a global mechanism to measure the uptake and impact of co-packaging.
CHTF wishes to institutionalize the monitoring of the uptake of co-packaged ORS/Zinc. One way of doing this would be to update the appropriate DHS template questionnaire. This will require CHTF to engage with the next DHS review process which was due to start in Sep-2023. We now believe this will take place in early 2025 and we are working to ensure we are included in this review process.
Our focus will be on the current Question 615 in the Woman’s Questionnaire:
Q. 615: SPECIAL FLUIDS AND ZINC
Women are asked if they gave a child with diarrhea fluid made from a packet of oral rehydration salts (ORS) such as [LOCAL NAME FOR ORS PACKET], a pre-packaged ORS liquid such as [LOCAL NAME FOR PRE-PACKAGED ORS LIQUIDS], zinc tablets or syrup, or [a government- recommended homemade fluid]. Read out each item and record the answer given for each one. (Source, Page 103.)We’d like to change this, or split it into two questions to establish the uptake of co-packaging.

ORS & ZINC STATUS AROUND THE WORLD.
We are collecting data submitted by members (historically ORSZCA and current CHTF) on the ORS and Zinc status in their country, or a country they are familiar with. This dataset seeks to answer the most frequently asked question, that no one can answer, which is “What is the current status of ORS and Zinc across LMICs?” Please add to this dataset using this questionnaire or by leaving a comment at the bottom of the individual country page. It takes less than 10 minutes to submit the questionnaire and helps us provide a unique resource for would-be donors and strategists.
Click here for the English questionnaire Cliquez ici pour le questionnaire
 Resources
Resources
Share a resource with us ›Tell us how you use the hub ›More ORS/Zinc Co-packing resources ›